BOSTON, Nov 19, 2020 /PRNewswire/ — Allos Pharma Inc, a late-stage pharmaceutical company developing therapeutics for neurodevelopmental disorders, has announced the exclusive license rights on arbaclofen in Fragile X Syndrome (FXS). The company is preparing the regulatory path for market authorization. Allos Pharma was founded to translate breakthrough discoveries in neurobiology into innovative drug treatments that improve the lives of patients and families with Fragile X Syndrome, autism, and other disorders of brain development.
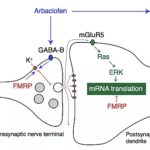
“Arbaclofen is the active enantiomer of baclofen, a GABA-B receptor agonist. Animal research confirmed that arbaclofen can correct core pathophysiology in Fragile X that arises from dysregulation of neuronal protein synthesis and hyperexcitability” says Professor Mark Bear and co-founder of Allos Pharma.
“Statistically significant and clinically meaningful efficacy was observed in a prior Phase 3 trial conducted by Seaside Therapeutics in humans with Fragile X Syndrome.” says Randy Carpenter, MD, and co-founder of Allos Pharma.
“The Fragile X professional field is excited about the opportunity to further explore arbaclofen in fragile X syndrome. The Fragile X community deserves this follow up work and the NFXF fully supports the professionals who have continually advocated for the access of the past trial data to inform future work to make arbaclofen available to individuals with Fragile X Syndrome. NFXF supports the future work of Allos Pharma and looks forward to working in partnership to better the lives of individuals with Fragile X Syndrome and their families.” says Linda Sorensen, Executive Director of the National Fragile X Foundation (NFXF).
“We are delighted that Drs. Bear and Carpenter and the rest of the Allos team are moving ahead with arbaclofen to treat core symptoms in children with Fragile X. Many, many families have been waiting eagerly for this news!” says Katie Clapp, President and co-founder of FRAXA Research Foundation.
About Allos Pharma, Inc.
Headquartered in Cambridge, Massachusetts, Allos Pharma is a late-stage pharmaceutical company developing therapeutics for neurodevelopmental disorders. Our mission is to translate breakthrough discoveries in neurobiology into innovative drug treatments that improve the lives of patients and families with Fragile X Syndrome, autism, and other disorders of brain development. Co-founders Mark Bear and Randy Carpenter previously founded Seaside Therapeutics and advanced arbaclofen through the Phase 3 trials in Fragile X Syndrome. This domain knowledge and expertise will be directly leveraged to advance arbaclofen through NDA approval. For more information visit www.allospharma.com