CAMBRIDGE, Mass., May 23, 2023 /PRNewswire/ — Allos Pharma Inc. (“Allos”), a biopharmaceutical company specializing in the development of treatments for rare neurological disorders, announced today that it has held a meeting with the U.S. Food and Drug Administration (FDA) to optimize the design of their Phase 3 trial. This trial is designed to support a New Drug Application (NDA) to address Fragile X Syndrome, for which there is no approved medicine today.
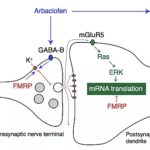
Allos’ investigational drug, arbaclofen, targets the molecular pathophysiology of Fragile X Syndrome and has demonstrated considerable efficacy in an FDA-compliant Phase 3 trial. The robust efficacy observed in children provides clarity on effective dosage, inclusion criteria, trial duration, safety, and endpoints, increasing confidence that the pivotal Phase 3 trial will yield positive results.
During the FDA meeting, Allos received invaluable and constructive feedback that is being leveraged with input from the Clinical Trial Committee of the National Fragile X Foundation (NFXF) and will be incorporated into the design of the Phase 3 trial.
Hilary Rosselot, Executive Director of the National Fragile X Foundation (NFXF) comments that “the Allos team has made significant progress in developing arbaclofen. The expert clinician members of our Clinical Trial Committee have provided input and guidance to optimize the design of the clinical trial and enthusiastically support performing this study. NFXF looks forward to working in partnership with Allos to better the lives of individuals with Fragile X Syndrome and their families.”
“We support the dedicated work of Drs. Bear and Carpenter and the rest of the Allos team to move ahead with arbaclofen to treat core symptoms in children with Fragile X. Many, many families are waiting eagerly to have access to arbaclofen again!” says Katie Clapp, President and co-founder of FRAXA Research Foundation.
“We are extremely pleased with the outcome of the meeting with the FDA and grateful for their engagement, guidance and support as we advance our Phase 3 trial for Fragile X Syndrome,” said Dr. Randall Carpenter, MD, co-founder of Allos Pharma Inc. “We remain committed to providing a new treatment option for patients and families affected by this debilitating disorder, and believe that arbaclofen has the potential to make a significant, positive impact on their lives.”
Additional data analyses suggested by the FDA bolster confidence that the statistically significant improvements observed in the Phase 3 trial are clinically meaningful. Specifically, anchor-based, within-patient responder analyses were performed on data collected in the Phase 3 studies previously conducted by Seaside Therapeutics. Significantly more children treated with arbaclofen had a clinically meaningful magnitude of improvement (P=0.015) than the placebo control group. These results were presented at 18th NFXF International Fragile X Conference July 14-17th 2022 . The robust dose-response observed on the clinical outcome assessments in this trial further support the likelihood that efficacy will be replicated in the confirmatory trial.
About Allos Pharma Inc.
Allos Pharma Inc. is a biopharmaceutical company dedicated to the development of treatments for rare neurological disorders. Allos’ investigational drug, arbaclofen, is currently in Phase 3 clinical development for the treatment of Fragile X Syndrome.
For more information, please visit Allos Pharma Inc.’s website at www.allospharma.com