CAMBRIDGE, Mass., July 14, 2022 /PRNewswire/ — Allos Pharma Inc (“Allos”), a late-stage pharmaceutical company developing therapeutics for neurodevelopmental disorders, announces presentation of “Arbaclofen produces clinically meaningful improvements in individuals with Fragile X” at the 18th NFXF International Fragile X Conference, July 14-17th, 2022. Allos Pharma was founded to translate breakthrough discoveries in neurobiology into innovative drug treatments that improve the lives of patients and families with Fragile X Syndrome, autism, and other disorders of brain development.
Meaningful change thresholds (MCTs) were applied for improvement on three subscales of the Fragile X-specific aberrant behavior checklist (ABCFX), Irritability, Social Avoidance and Socially Unresponsive/Lethargic to assess efficacy in the Phase 3 trial in children, aged 5-11. Clinically meaningful magnitudes of improvement were observed on all three ABCFX subscales for 45% of individuals receiving the high dose of arbaclofen vs 4% of those treated with placebo (P=0.00003), clearly demonstrating the efficacy of arbaclofen in individuals with Fragile X. “These findings comport remarkably well with experiences reported by clinicians involved in the clinical trials, who reported that ~50% of those receiving arbaclofen showed clear and substantial improvements.” Says Dr. Elizabeth Berry-Kravis, MD, Rush University Medical Center.
“We are presenting new analyses of the two phase 3 trials for arbaclofen in Fragile X Syndrome. In addition to the efficacy previously reported, statistically significant efficacy was demonstrated using the current FDA standards for within-subject responder analyses. This represents an important milestone in our work with the regulatory agencies to bring arbaclofen to market” says Randy Carpenter, MD, and co-founder of Allos.
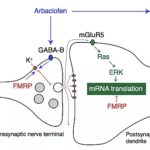
“This result supports our hypothesis that arbaclofen correction of core pathophysiology in Fragile X Syndrome arising from dysregulation of neuronal protein synthesis and hyperexcitability will confer broad therapeutic benefit. Combining the new results with the existing literature forms a positive picture of the efficacy of arbaclofen.” says Professor Mark Bear and co-founder of Allos.
About Allos Pharma, Inc.
Headquartered in Cambridge, Massachusetts, Allos Pharma is a late-stage pharmaceutical company developing therapeutics for neurodevelopmental disorders. Our mission is to translate breakthrough discoveries in neurobiology into innovative drug treatments that improve the lives of patients and families with Fragile X Syndrome, autism, and other disorders of brain development. Co-founders Mark Bear and Randy Carpenter previously founded Seaside Therapeutics and advanced arbaclofen through the Phase 3 trials in Fragile X Syndrome. This domain knowledge and expertise will be directly leveraged to advance arbaclofen through NDA approval. For more information visit www.allospharma.com.